Selecting the right power supply for medical equipment can be daunting, as it's crucial to choose a properly qualified option. This article aims to clear up some of the confusion, offering a concise overview of key terminology and important factors to consider when dealing with medical power supplies.
Murata products for Medical Applications

Environmental changes surrounding healthcare affect personal healthcare devices used for daily health management, with such products becoming more compact and gaining the ability to connect to the cloud. Discover how Murata’s products contribute to:
- The ability to create more compact products and exercise more design freedom thanks to the availability of compact general-purpose components (capacitors, inductors, etc.)
- The ability to easily create daily health monitoring products with network connectivity due to the availability of Bluetooth® Smart Modules, wireless communication modules supporting near-field communication (NFC), and much more.
What is a Medical-Rated AC-DC Power Supply?
A medical-rated AC-DC power supply is one that has been evaluated and certified according to the latest medical standards, such as IEC 60601-1. This standard includes specific requirements for "Means of Protection (MOP)," especially concerning the safety of the patient. The key protections are:
- MOOP (Means of Operator Protection)
- MOPP (Means of Patient Protection)

Figure 1
Figure 1 illustrates an Insulation Diagram of a Murata Power Solutions (MPS) medical-rated power supply, highlighting the different levels and types of protection in various areas:
- Area “A” = 1 x MOOP
- Area “B” = 1 x MOPP
- Area “C” = 2 x MOPP
- Area “D” = 1 x MOPP
- Area “E” = 1 x MOPP
- Area “F” = 1 x MOPP
This consideration is crucial in the final system application. If the power supply output connects to the patient without 1MOPP isolation from the power supply, it may lead to potential system-level non-compliance.
End users can be confident that the MOP built into any medical-approved Murata Power Solutions (MPS) power supply has been evaluated by a third-party accredited safety agency. This ensures one less concern for the end user. However, it remains necessary to determine which level of MOP is suitable for the intended application.
Figure 2 (extracted from IEC60601-1 Ed 3) presents the decision-making process in a flowchart format, guiding the assessment and determination of the type of protection (MOP) required for any medical equipment application:

Figure 2
The first decision box addresses the "APPLIED PART"; what does this mean exactly? An Applied Part is a component that may come into contact with the patient. This is a crucial decision for the end user, based on the following three Types of Applied Parts versus the intended application:
- Type B (Body): This type can be in contact with the patient’s body and may be connected to earth. The associated risk is excessive patient leakage current. Examples include LED lighting, medical lasers, MRI/CT body scanners, hospital beds, and phototherapy equipment.
- Type BF (Body Floating): This type also contacts the patient’s body, but the patient is not directly connected to earth, thus considered "floating" relative to earth. The risk here is also excessive patient leakage current. Examples include blood pressure monitors, incubators, and ultrasound equipment.
- Type CF (Cardiac Floating): This type is for equipment intended for direct cardiac (or bloodstream) application, requiring extremely low patient leakage current limits. Examples include dialysis and surgical equipment.
Note that power supplies are not medical equipment or applied parts and should not be directly connected to a patient. If none of these types apply, the intended application does not necessarily require an Applied Part rated power supply, although some form of medical certification may still be needed.
Once this decision is made, research into suitable products can be conducted to meet both the operational requirements of the intended application and the appropriate level of MOP.
Leakage Currents and How They Influence the Selection of the Power Supply
Leakage currents are another often confusing parameter. IEC 60601-1 Ed 3 defines three main types of leakage current:
- Earth Leakage Current
- Enclosure (Touch) Current
- Patient Leakage Current
Leakage (unwanted) current can be AC, DC, or a combination of both, originating from the input (AC source side) and/or the output side of the power supply.
Earth Leakage Current
Figure 3 illustrates the current that flows from the AC input source, generally via capacitance within the power supply, through the earth conductor (Protective Earth). This capacitance can be both parasitic (stray) and intentionally placed between the AC source connections (live and neutral) to earth to reduce EMI emissions.
There is no risk if the earth connection is solid, as no current can flow through the patient/operator to earth, even if they are directly in contact with the enclosure. Under normal conditions, the allowable current is 5mA, and for Single Fault Conditions (SFC), it is allowed to be 10mA.
Figure 3 also shows the position of the EMI "Y" capacitors on a Murata Power Solutions PQC250 open frame power supply.

Figure 3

Enclosure (Touch) Leakage Current
Figure 4 illustrates the current that would flow if the patient (or operator) were connected to earth and in direct contact with the enclosure. Under normal conditions, the allowable current is very low at 100μA, and for Single Fault Conditions (SFC), it is allowed to be up to 500μA.

Figure 4
Patient Leakage Current
Figure 5 depicts the current flowing between a piece of medical equipment and through the patient to earth. Under normal conditions (NC), the allowable current is 100μA. Under Single Fault Conditions (SFC), this allowable current increases to 500μA.

Figure 5
Leakage current can also flow from another source i.e., another item of medical equipment, connected to the Patient, to earth (as shown in Figure 6).

Figure 6
There is a fourth leakage current mentioned in IEC 60601 Ed 3, however, this is out of the scope of this brief.
Grounding: Class I and Class II Equipment
In IEC 60601-1 Ed 3, Class I and Class II equipment are defined in terms of protection against electric shock. Class I: Equipment in this class is designed to have a Protective Earth (PE) connection. The insulation system between the primary components and all metal or internal metal parts is basic. This means there must be a PE connection on the metal enclosure of the equipment. Devices intended for Class I connection to the utility typically feature three connector terminals, one of which is designated as the PE contact (refer to Figure 7).
Class II: Equipment in this class is not intended to have a PE connection. Therefore, the insulation between the primary components and all metal or internal metal parts must be double or reinforced. Devices intended for Class II connection to the utility generally have a two-terminal connector (see Figure 8). However, a three-terminal connector may be used for grounding purposes related to EMI, but this is not considered a PE contact.
Class II equipment typically has a non-conductive enclosure (plastic). Metal enclosures are permissible for Class II products provided they offer double insulation (2xMOOP) from the primary to PE/Ground and to the end user.
Ultimately, the end user must determine the required insulation class based on:
- Whether the enclosure is conductive (metal).
- If a PE connection is available; in such cases, selecting a power supply with an appropriate connector is crucial.
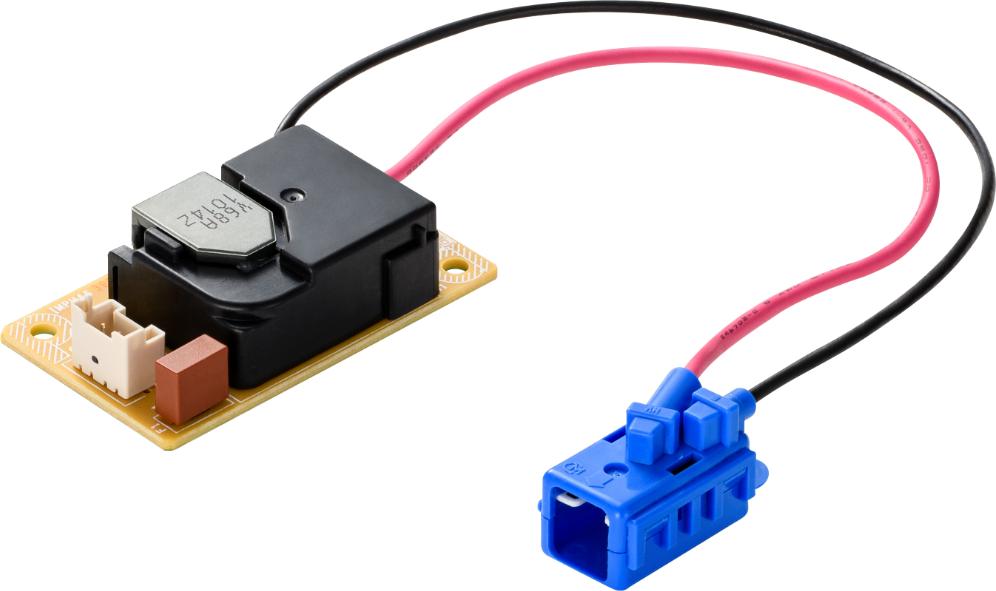
Examples of Murata Power Solutions Products for Class I implementation:

Figure 7 - PQU650M-xxP: Three pin header with integral PE

Figure 8 - PQC250 Series: Two pin header with separate PE terminal.
Summary
This article has provided an overview of common considerations when selecting a suitable power supply for medical applications. It begins with a review of Means of Protection for both patients and operators, emphasizing their impact on power supply selection for different types of medical equipment.
After determining the equipment's classification as an "Applied Part," the article underscores that while power supplies themselves are not Applied Parts, they significantly influence medical equipment deployment.
Leakage current is highlighted as a critical parameter influencing power supply choice, with a detailed examination of its types and implications for medical equipment.
Lastly, grounding considerations are explored in relation to power supply selection.